CHANNELS

Group Leader
Prof. Dr. Thomas Benzing
+49 221 478-4480
thomas.benzing@uk-koeln.de
TEAM MEMBERS
Linus Butt, MD
Robert Hahnfeldt
Amrei Mandel
Amer Ramdedovic
David Unnersjö-Jess, PhD
Thomas Benzing
Podocyte signaling and glomerular biology
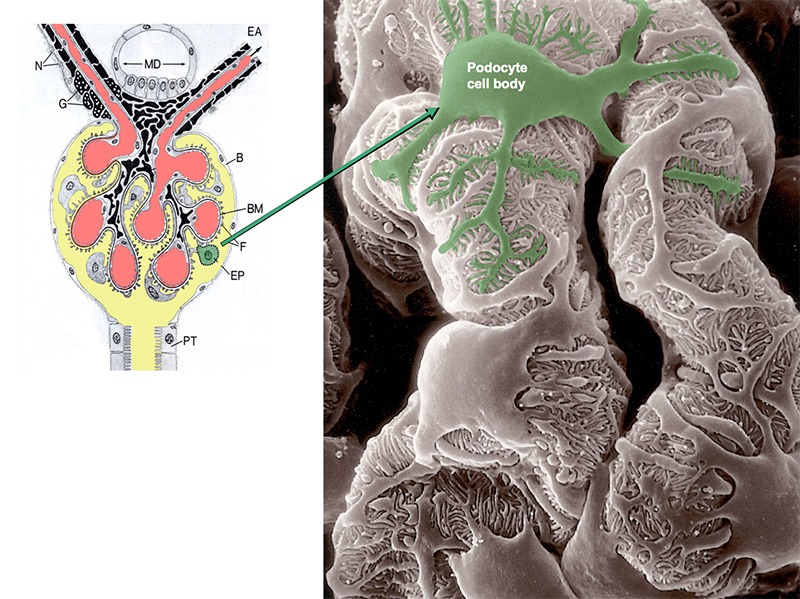
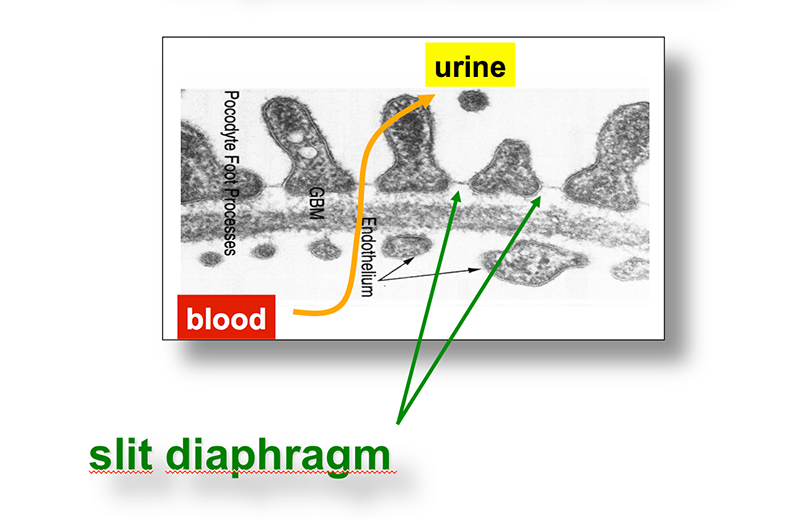
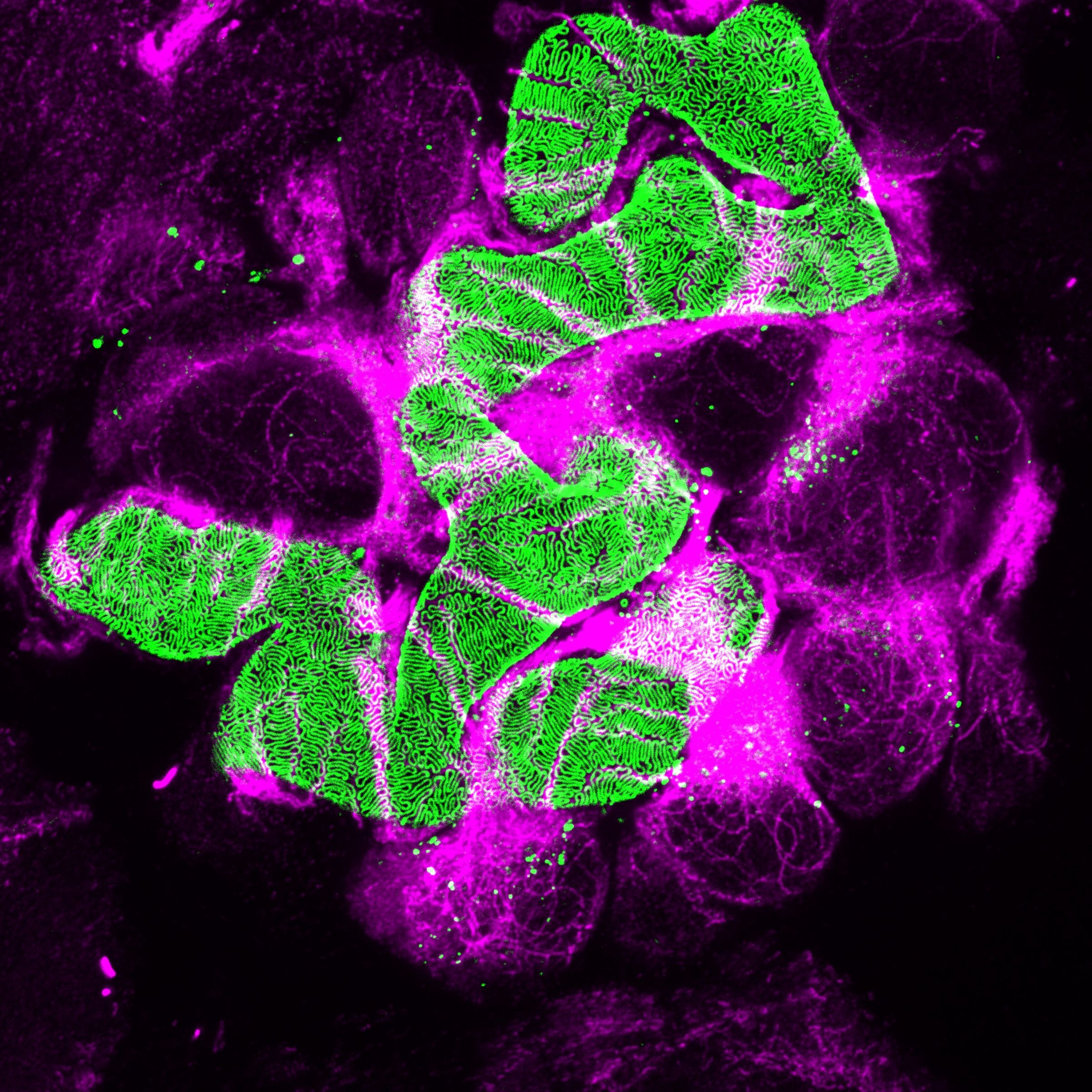
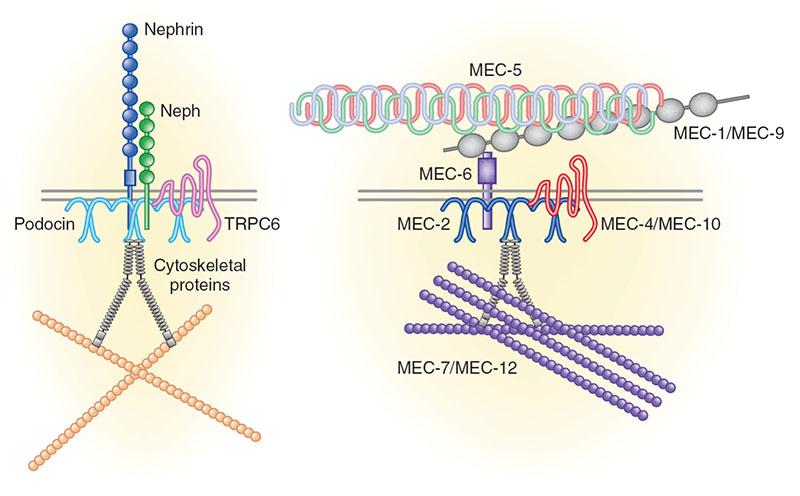
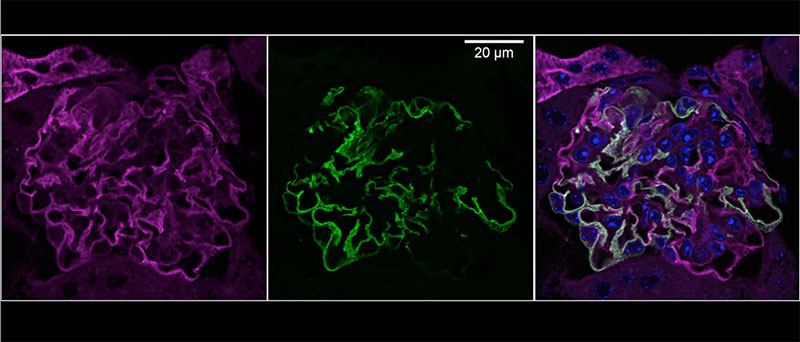
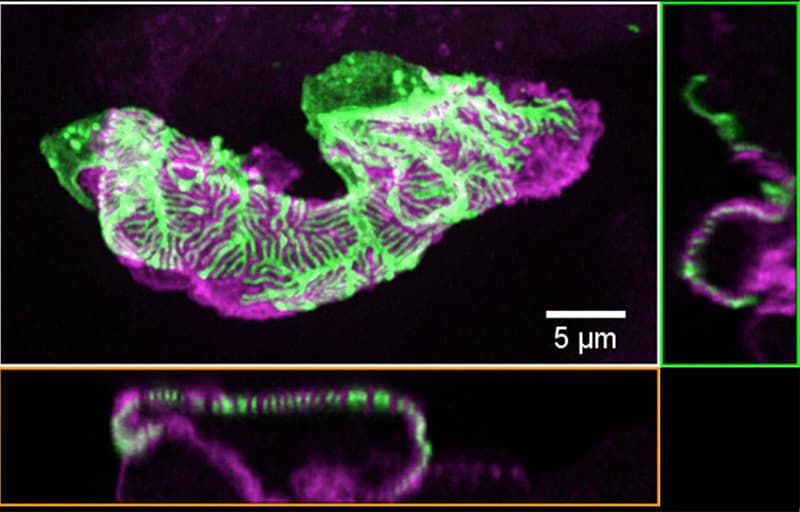
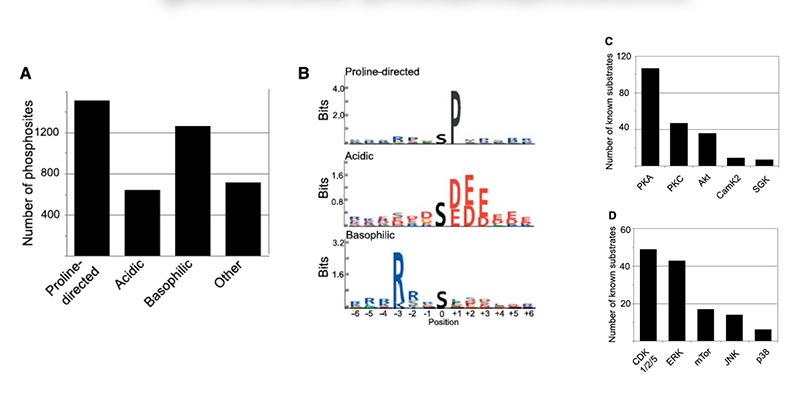
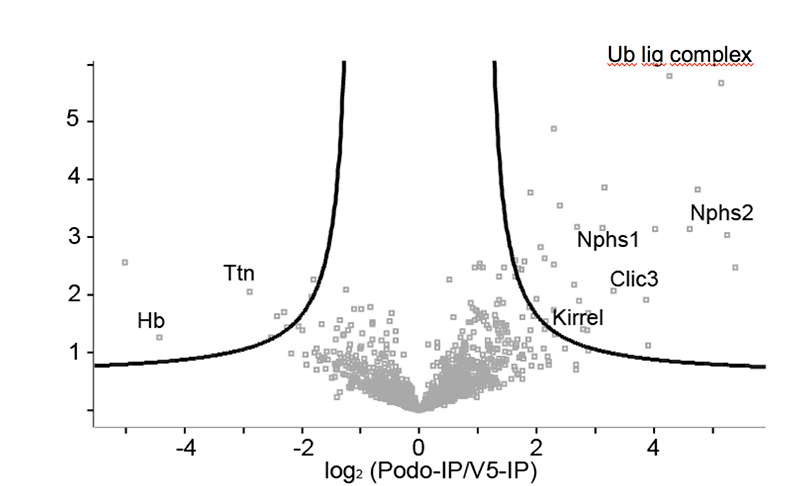
Chronic kidney disease (CKD) is becoming an increasingly prevalent condition affecting almost 10% of the population in Western societies. The majority of kidney diseases that progress to CKD start in the glomerulus, the renal filtration unit, as a consequence of a very limited capacity of glomeruli for regeneration and the limited ability of terminally differentiated glomerular podocytes for self-renewal. Postmitotic podocytes are essential components of the three-layered glomerular filtration barrier of the kidney, and we have demonstrated that podocyte function is regulated through signaling at a highly specialized cell junction, called the slit diaphragm. Over the past decade, my team has pioneered the concept that podocytes are master regulators of the glomerular microcirculation/filtration. Using methods of advanced molecular biology and phosphoproteomics as well as genetically engineered animals we deciphered signaling networks that are controlled through slit diaphragm-associated protein complexes and showed that these signaling networks are essential for podocyte viability and function and the physiology of kidney filtration. Moreover, we showed that podocytes are acutely mechanosensitive through mechanisms involving the ion channel TRPC6 and the PHB-domain protein Podocin. Our work greatly benefits from insight derived in the model organisms C. elegans, Drosophila melanogaster as well as genetically engineered mice.
Recently, we were able to provide the first experimentally validated model of the function of the kidney filter. This groundbreaking research, which will undoubtedly change medical textbook knowledge, resulted from interactions with biomedical engineers at Massachusetts Institute of Technology and Boston University, computational scientists in Cologne, and elsewhere. The approach may serve as an example of a research direction into computational approaches that we will also follow. The research team is closely connected to the clinical department that I am chairing, and that is affiliated with a large clinical research unit. Here we aim to translate the findings in the lab to new treatment options in patients suffering from kidney disease. The group greatly benefits from close interactions at the Cluster of Excellence on Cellular Stress Responses in Aging Associated Diseases (CECAD) and the Center for Molecular Medicine Cologne (CMMC), as well as the Systems Biology of Ageing network (Sybacol).
SELECTED PUBLICATIONS
Unnersjo-Jess, D., Butt, L., Hohne, M., Witasp, A., Kuhne, L., Hoyer, P.F., Patrakka, J., Brinkkotter, P.T., Wernerson, A., Schermer, B., Benzing, T., Scott, L., Brismar, H., and Blom, H. (2021) A fast and simple clearing and swelling protocol for 3D in-situ imaging of the kidney across scales. Kidney Int, 99(4): 1010-1020.
Butt, L., Unnersjo-Jess, D., Hohne, M., Schermer, B., Edwards, A., and Benzing, T. (2021) A mathematical estimation of the physical forces driving podocyte detachment. Kidney Int, 100(5): 1054-1062.
Benzing, T. and Salant, D. (2021) Insights into Glomerular Filtration and Albuminuria. N Engl J Med, 384(15): 1437-1446.
Butt, L., Unnersjo-Jess, D., Hohne, M., Edwards, A., Binz-Lotter, J., Reilly, D., Hahnfeldt, R., Ziegler, V., Fremter, K., Rinschen, M.M., Helmstadter, M., Ebert, L.K., Castrop, H., Hackl, M.J., Walz, G., Brinkkoetter, P.T., Liebau, M.C., Tory, K., Hoyer, P.F., Beck, B.B., Brismar, H., Blom, H., Schermer, B., and Benzing, T. (2020) A molecular mechanism explaining albuminuria in kidney disease. Nat Metab, 2(5): 461-474.
Rinschen, M.M., Godel, M., Grahammer, F., Zschiedrich, S., Helmstadter, M., Kretz, O., Zarei, M., Braun, D.A., Dittrich, S., Pahmeyer, C., Schroder, P., Teetzen, C., Gee, H., Daouk, G., Pohl, M., Kuhn, E., Schermer, B., Kuttner, V., Boerries, M., Busch, H., Schiffer, M., Bergmann, C., Kruger, M., Hildebrandt, F., Dengjel, J., Benzing, T., and Huber, T.B. (2018) A Multi-layered Quantitative In Vivo Expression Atlas of the Podocyte Unravels Kidney Disease Candidate Genes. Cell Rep, 23(8): 2495-2508.
Rinschen, M.M., Hoppe, A.K., Grahammer, F., Kann, M., Volker, L.A., Schurek, E.M., Binz, J., Hohne, M., Demir, F., Malisic, M., Huber, T.B., Kurschat, C., Kizhakkedathu, J.N., Schermer, B., Huesgen, P.F., and Benzing, T. (2017) N-Degradomic Analysis Reveals a Proteolytic Network Processing the Podocyte Cytoskeleton. J Am Soc Nephrol, 28(10): 2867-2878.
Ising, C., Bharill, P., Brinkkoetter, S., Brahler, S., Schroeter, C., Koehler, S., Hagmann, H., Merkwirth, C., Hohne, M., Muller, R.U., Fabretti, F., Schermer, B., Bloch, W., Kerjaschki, D., Kurschat, C.E., Benzing, T., and Brinkkoetter, P.T. (2016) Prohibitin-2 Depletion Unravels Extra-Mitochondrial Functions at the Kidney Filtration Barrier. Am J Pathol, 186(5): 1128-39.
Brinkkoetter, P.T., Ising, C., and Benzing, T. (2013) The role of the podocyte in albumin filtration. Nat Rev Nephrol, 9(6): 328-36.
